

This causes air to flow into the lungs (from high pressure to low pressure). The increase in volume leads to a decrease in pressure (Boyle’s law). When you inhale, your diaphragm and intercostal muscles (the muscles between your ribs) contract, expanding your chest cavity and making your lung volume larger. Lungs are made of spongy, stretchy tissue that expands and contracts while you breathe. Your lungs take in gas that your body needs (oxygen) and get rid of waste gas (carbon dioxide). How does it work? It turns out that the gas laws apply here.
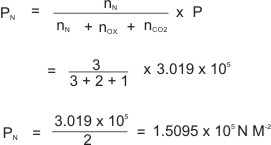
What do you do about 20 times per minute for your whole life, without break, and often without even being aware of it? The answer, of course, is respiration, or breathing. V graph in Figure 5Ĭomment on the likely accuracy of each method.Ĭhemistry in Action: Breathing and Boyle’s Law Where ∝ means “is proportional to,” and k is a proportionality constant that depends on the identity, amount, and volume of the gas.įor a confined, constant volume of gas, the ratio \frac vs. Under either name, it states that the pressure of a given amount of gas is directly proportional to its temperature on the kelvin scale when the volume is held constant. Because of this, the P– T relationship for gases is known as either Amontons’s law or Gay-Lussac’s law. Guillaume Amontons was the first to empirically establish the relationship between the pressure and the temperature of a gas (~1700), and Joseph Louis Gay-Lussac determined the relationship more precisely (~1800). (Measurements cannot be made at lower temperatures because of the condensation of the gas.) When this line is extrapolated to lower pressures, it reaches a pressure of 0 at –273 ☌, which is 0 on the kelvin scale and the lowest possible temperature, called absolute zero. For a constant volume and amount of air, the pressure and temperature are directly proportional, provided the temperature is in kelvin. We will consider the key developments in individual relationships (for pedagogical reasons not quite in historical order), then put them together in the ideal gas law.įigure 3. Eventually, these individual laws were combined into a single equation-the ideal gas law-that relates gas quantities for gases and is quite accurate for low pressures and moderate temperatures. Although their measurements were not precise by today’s standards, they were able to determine the mathematical relationships between pairs of these variables (e.g., pressure and temperature, pressure and volume) that hold for an ideal gas-a hypothetical construct that real gases approximate under certain conditions. Use the ideal gas law, and related gas laws, to compute the values of various gas properties under specified conditionsĭuring the seventeenth and especially eighteenth centuries, driven both by a desire to understand nature and a quest to make balloons in which they could fly (Figure 1), a number of scientists established the relationships between the macroscopic physical properties of gases, that is, pressure, volume, temperature, and amount of gas.Identify the mathematical relationships between the various properties of gases.
#PRESSURE CHEMISTRY CALCULATOR FREE#
PS: I went quickly through the procedure without really explaining why at each step, if you are curious feel free to post a reply and I can follow up.By the end of this section, you will be able to: roughly then we get 64 atomic mass units per molecule, which implies: S has an atomic weight of 32.065 while each oxygen contributes 15.999. Thus we consult the periodic table to see how many protons and neutrons are present in one molecule of SO2. Atomic mass units are the mass of a proton, or neutron, which is where basically all the mass for atoms and molecules comes from. First we find the number of atomic mass units within one molecule of SO2, then we simply take that to be the number of grams in a mol of SO2. Conveniently, the number of molecules in a mol was chosen specifically to make this conversion easy.
To find the mass in grams we need to know how many grams a mol of SO2 weighs. To find the number of moles we just rearrange: The units are liter atmospheres per mol per degree kelvin, so we will need to convert our temperature from Celsius to kelvin by adding 273 to it. Where P is pressure, V is volume, T is temperature, n is number of moles and R is a constant equal to:


 0 kommentar(er)
0 kommentar(er)
